The Blue Standard: More Than Color in Medical Device Security
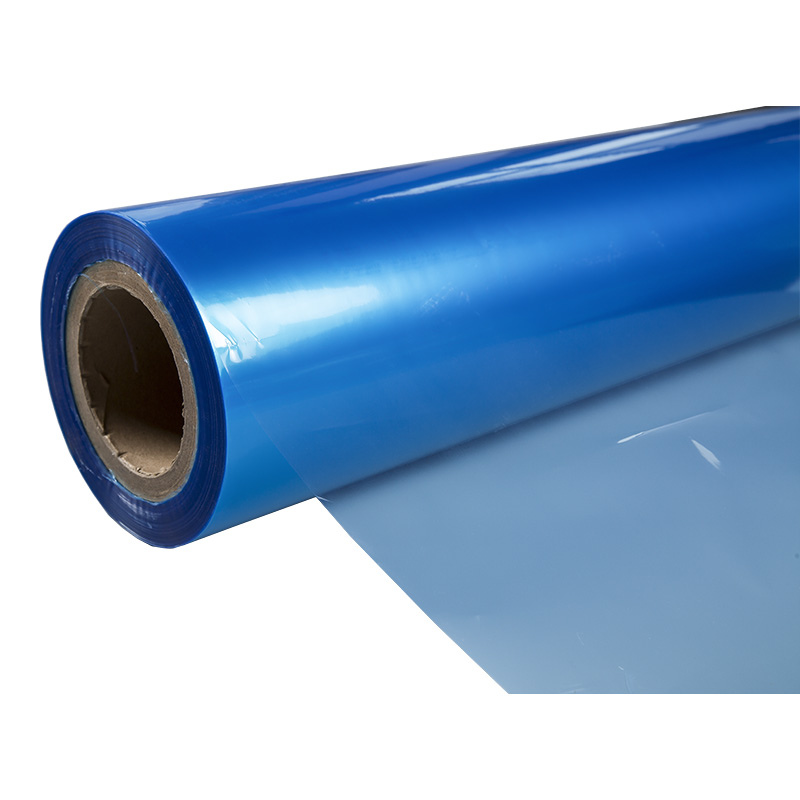
In the high-stakes world of medical device manufacturing, the Blue Medical Device Packaging Film Roll is far more than a colored plastic sheet. It is the primary component of the sterile barrier system (SBS), a critical line of defense that maintains a device's sterility from the point of manufacture to the point of use in a surgical suite. The iconic blue hue serves practical purposes: it provides a consistent, non-glare background for automated optical inspection (AOI) systems to detect particulate contamination and offers high contrast against most operating room materials. Selecting the correct film is a complex decision involving material science, regulatory compliance, and production process compatibility. This guide provides a detailed framework for packaging engineers, quality assurance professionals, and procurement specialists to evaluate and specify film that ensures patient safety, product integrity, and regulatory approval.
Material Science and Performance Decoded
Understanding the fundamental properties of packaging films is essential for making an informed selection. Performance is dictated by the base material, its structure, and its coating.
The most common base materials are polyethylene and Tyvek (a brand of flash-spun high-density polyethylene fibers). Polyethylene films, used in thermoforming, offer excellent clarity, flexibility, and a range of sealant properties. Tyvek, a non-woven material, provides an exceptional microbial barrier due to its labyrinthine fiber structure and is highly resistant to tears and punctures. Beyond the base, films are engineered with specific performance layers. A critical property is the sealant layer, which must create a strong, hermetic seal with the chosen lidding material when heat is applied. The film's coefficient of friction (COF) is vital for machinability, affecting how it unwinds and feeds into vertical or horizontal form-fill-seal equipment. Key performance data from suppliers should detail tensile strength, elongation, moisture vapor transmission rate (MVTR), and, crucially, validated peel strength after aging and sterilization.
- Technical Insight: Always request and review the film's DSC (Differential Scanning Calorimetry) curve from the supplier. This graph shows the material's melting and crystallization points, which are critical for dialing in the precise heat, pressure, and dwell time parameters on your sealing equipment to achieve consistent, reliable seals without burning or weakening the film.
Application-Driven Selection Guide
The "right" film is entirely dependent on the device it protects, the required sterilization method, and the intended shelf life. Matching film properties to these application demands is the core of the specification process.
1. Protecting Surgical Instrument Kits: The Foundation of Sterility
For surgical kit packaging, the film must be a proven sterile blue medical grade film roll. These kits contain multiple components and are subject to rigorous handling. The film must withstand the chosen sterilization modality—whether Ethylene Oxide (EtO), Gamma, or E-beam—without becoming brittle, discolored, or experiencing a significant change in seal strength. It must also provide excellent clarity for kit content visibility and have consistent mechanical properties to ensure clean, predictable tear initiation and propagation when the nurse opens the package at the sterile field. The film's coating must be compatible with the lidding material to create a robust seal that maintains integrity through distribution and storage.
2. Long-Term Implant Security: The Highest Barrier Demands
Implants like orthopedic joints or cardiovascular stents require packaging that can maintain a sterile environment for many years. This is the domain of a high barrier blue Tyvek film roll for long-term implant packaging. Tyvek's non-woven structure provides an almost absolute barrier to microbial ingress, which is paramount for long shelf-life products. Its high tensile strength protects against punctures from sharp implant features during handling and transit. While Tyvek is permeable to gases (allowing for EtO sterilization and aeration), its MVTR is low, helping to control the package's internal microenvironment. For even more demanding applications, foil-based laminates or films with special ceramic coatings are used to achieve near-zero gas transmission.
3. Matching the Manufacturing Process: Thermoforming Compatibility
Selecting a blue polyethylene film roll for medical device thermoforming requires a deep understanding of the thermoform-fill-seal (TFFS) process. The film must have the correct thermoforming window—the range of temperatures at which it can be heated and stretched into a cavity mold without thinning excessively, tearing, or losing its barrier properties. The film's gauge (thickness) and consistency are critical, as variations will lead to uneven wall thickness in the formed tray, creating weak spots. The sealant layer on the film's flange must be perfectly matched to the lidding material to create a peelable seal that is strong yet allows for aseptic presentation.
4. The Cornerstone of Compliance: Adhering to Global Standards
For any medical device, using an ISO 11607 compliant blue medical packaging film roll is not optional; it's a regulatory imperative. ISO 11607-1 specifies the requirements for materials, preformed sterile barrier systems, and packaging systems. Compliance means the film supplier has conducted and can provide evidence of necessary testing, including:
- Biological Evaluation (per ISO 10993-1) to ensure biocompatibility.
- Physical and Chemical Testing to validate performance properties.
- Sterilization Validation data proving the film can withstand the specified sterilization process without functional degradation.
Your device's packaging validation (per ISO 11607-2) is built upon the foundation of using compliant, characterized materials from a qualified supplier.
5. Integrating Information: Printing and Traceability
A growing trend is the use of custom printed blue film roll for medical device instructions for use (IFU) and traceability data. Printing graphics, symbols, handling instructions, or even batch information directly onto the forming web streamlines the packaging process, reduces label application errors, and enhances the professional appearance. The printing must use biocompatible inks that do not migrate, affect seal integrity, or off-gas during sterilization. Common methods include flexographic and digital printing, with the latter being ideal for high-variable-data applications like unique device identifiers (UDIs).
| Application Focus | Surgical Instrument Kits | Long-Term Implants | Thermoformed Devices |
| Primary Film Type | Coated Medical Grade PE Film | Tyvek or High-Barrier Laminate | Multi-layer PE Co-extrusion |
| Critical Performance Need | Sterilization Stability, Clean Tear, Clarity | Absolute Microbial Barrier, Long-Term Aging Stability | Wide Thermoforming Window, Consistent Gauge, Seal Integrity |
| Key Selection Spec | Seal Strength Post-Sterilization, Tear Propagation Resistance | MVTR, Microbial Barrier Efficacy (ASTM F1608) | DSC Melting Point, Modulus, Minimum Forming Ratio |
Supplier Qualification and Risk Management
Choosing a film supplier is a critical partnership. A qualified supplier does more than sell material; they provide technical support, consistency, and regulatory assurance. The qualification process should include an audit of their quality management system (preferably ISO 13485 certified), a review of their change control procedures, and an assessment of their raw material sourcing and traceability. They should provide a full technical data sheet (TDS) and a regulatory data packet for each film grade. Establish clear agreements on notification timelines for any process or material changes, as such changes may require re-validation of your device packaging.
- Pro Tip for Validation: When qualifying a new film, always conduct real-time aging tests on your final packaged device in addition to accelerated aging. While accelerated aging (per ASTM F1980) is used to project shelf life, real-time data provides the definitive confirmation of packaging performance and is often looked upon favorably by regulatory reviewers.
FAQ
Why is blue the dominant color for medical packaging film?
The blue color serves several key functional purposes in a medical context. First, it provides a high-contrast, non-reflective background in the bright lights of an operating room, making it easier for surgical staff to see the contents of a kit. More importantly, the consistent blue hue is optimal for automated vision inspection systems on packaging lines. These systems can be calibrated to detect foreign particles, fibers, or defects against the uniform blue background with extremely high accuracy, ensuring no contaminated package proceeds to sterilization and distribution.
What is the real difference between "medical-grade" and standard packaging film?
The term "medical-grade" signifies that the film has been manufactured, tested, and documented to meet stringent regulatory requirements for direct or indirect patient contact. Key differentiators include: 1) Biological Compliance: It has been tested for biocompatibility (ISO 10993) to ensure it does not leach harmful chemicals. 2) Consistency and Purity: It is produced in a controlled environment with strict limits on additives and contaminants. 3) Full Traceability: Every batch can be traced back to its raw material sources and production parameters. 4) Validation Support: The supplier provides data to support its performance claims after sterilization and aging. Standard film lacks this guaranteed level of control and documentation.
How do I test if a film provides an adequate microbial barrier?
The standard test for microbial barrier properties is ASTM F1608. In this test, dry bacterial spores (usually *Bacillus atrophaeus*) are challenged against the material under specific conditions. For porous materials like Tyvek, the test measures the log reduction value (LRV), indicating how effectively the material blocks the passage of microbes. For non-porous films, the integrity of the seal is the primary microbial barrier, which is validated through whole-package integrity tests like dye penetration (ASTM F1929) or vacuum decay (ASTM F2338). Your film supplier should provide ASTM F1608 data for porous materials as part of their regulatory packet.
Can we use recycled content in medical device packaging film?
Generally, the use of post-consumer recycled (PCR) content is not permitted in primary medical packaging films that form the sterile barrier system. Regulatory bodies like the FDA require strict control and traceability of materials to ensure safety and performance. The unknown history and potential variability of PCR materials introduce an unacceptable risk of contamination, inconsistent properties, or failure to meet biocompatibility standards. The focus for sustainability in medical packaging is instead on design for recyclability, source reduction (using less material), and ensuring the packaging materials are clearly identifiable for proper disposal and potential recovery in dedicated streams.
What happens if the film's blue color shade varies between batches?
Significant color variation between film batches can be a serious operational problem. It can disrupt the calibration of automated optical inspection (AOI) systems on your packaging line, potentially causing false rejects or, worse, failing to detect actual contaminants. It may also raise concerns with hospital staff who associate specific shades with trusted brands. A professional film supplier will have tight color control specifications (using systems like CIE Lab values) and provide a certificate of analysis with each batch. Before approving a new lot for production, it is good practice to run a sample through your AOI system to verify it is correctly recognized.
 +86 139-6715-0258
+86 139-6715-0258 
 Monday to Friday 8 am. to 6 pm.
Monday to Friday 8 am. to 6 pm. 
 English
English 中文简体
中文简体





